Conduction
Conduction is the heat transfer mechanism in which thermal energy transfers
from one point to another through the interaction between the atoms or
molecules of the matter. Conduction occurs in solids, liquids, and gasses.
Conduction does not involve any bulk motion of matter. Gases transfer
heat by direct collisions between energetic molecules, and their thermal
conductivity is low compared to solids since they are dilute media. The
conduction of energy in liquids is the same as in gases except that the
situation is considerably more complex since the molecules are more closely
spaced and molecular force fields exert a strong influence on the energy
exchange in the collision process. Nonmetallic solids transfer heat by
lattice vibrations so there is no motion of the media as heat propagates
through. Metals are better conductors than nonmetals at normal temperatures
because they have free electrons that carry thermal energy.
The heat transfer by conduction obeys Fourier's law which states that
the rate of heat conduction Qconduction
is proportional to the heat transfer area (A) and the temperature
gradient (dT/dx), or:
Qconduction = - K
A (dT/dx)
where K,
the thermal conductivity, measures the ability of a material to conduct
heat. The units of K are W/m.oC
or (Btu/s)/in.oF. For
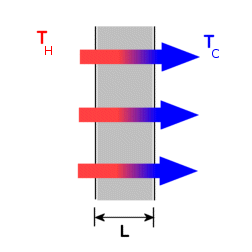
the planar layer shown below, the rate of heat conduction is given by,
Qconduction
= - K
A ( TH
- TC
)/L
This figure
shows range values for the thermal conductivity for liquids, nonmetallic
solids, and pure metals at normal temperature and pressure.
Temperature Dependence of the Thermal Conductivity
(K)
For most materials, K varies with temperature. It rises with temperature
in gases at low pressures, but it may rise or fall in metals or liquids.
The following table lists thermal conductivities (in W/m.oK)
versus temperature (in oK)
for selected materials:
|
Metal |
Temperature (oK) |
|
103 |
173 |
273 |
373 |
473 |
573 |
673 |
873 |
|
Stainless Steel |
|
|
|
15 |
17 |
19 |
21 |
25 |
|
Lead |
40 |
37 |
36 |
34 |
33 |
32 |
17 (liq.) |
20 (liq.) |
|
Platinum |
78 |
73 |
72 |
72 |
72 |
73 |
74 |
77 |
|
Zinc |
124 |
122 |
122 |
117 |
110 |
106 |
100 |
60 (liq.) |
|
Silicon |
856 |
342 |
168 |
112 |
82 |
66 |
54 |
38 |